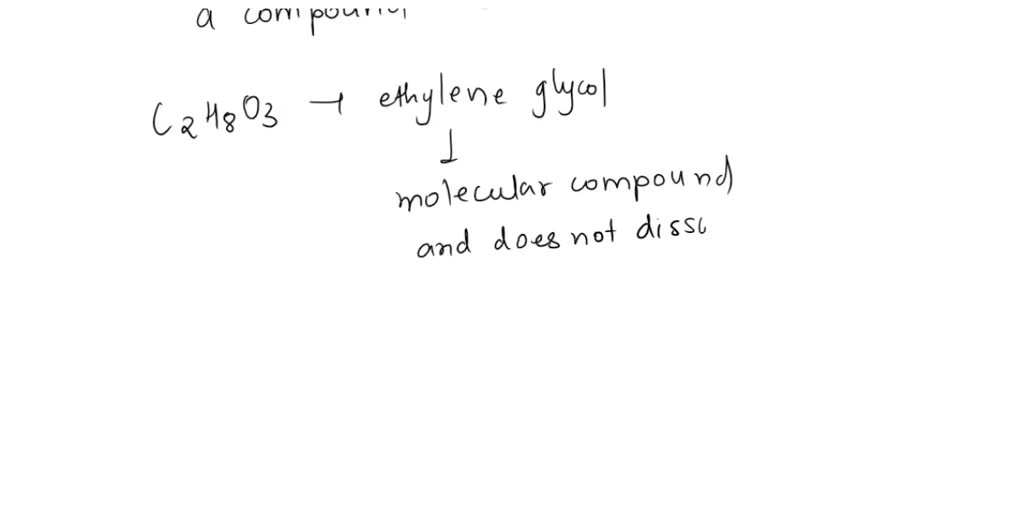Kcl sales vant hoff
The values of van t Hoff factors KCl NaCl and K 2SO 4 sales, The van t Hoff factor of a 0.005 M aqueous solution of KCl is 1.95 sales, SOLVED The van t Hoff factor for KCl is i 1.85 . What is the sales, The values of van t Hoff factors for KCl NaCl and K 2SO 4 respectively are 12 SOLUT sales, Van t Hoff factor is maximum in KCl 50 ionised K SO 40 ionised sales, A 0.6 aqueous solution of KCl mol. wt. 74.5 freezes at 0.2 sales, ii The value of van t Hoff factor i for aqueous KCl solution sales, The 15th one 15 If the van t Hoff factor of a 0 005M aqueous sales, SOLVED sales, SOLVED Determine the Van t Hoff factor for the following sales, the van t hoff factor of a 0 005 M aqueous solution of KCl is 1 96 sales, In which case van t Hoff factor i remains unchanged a PtCl4 sales, SOLVED 25. The observed and calculated molar mass of KCl is 38.75 sales, In which case vant Hoff factor is maximuma KCl 50 ionisedb sales, A 0.5 aqueous solution of KCl was found to freeze at 0.24 C. Calculate the Van t Hoff facto sales, Van t Hoff factor is maximum in KCl 50 ionised K SO 40 ionised sales, The values of Van t Hoff factors for KCl NaCl and K 2SO 4 sales, van t Hoff factor of a 0.5 w w aqueous solution of KCl sales, The values of van t Hoff factors for KCl NaCl and K 2SO 4 respectively are 12 SOLU sales, The van t Hoff factor of a 0.005 M aqueous solution of KCl is 1.95. The degree of ionisation of KCl is sales, The values of Van t Hoff factors for KCl NaCl and K 2SO 4 respectively are i 2 2 and 2 ii sales, The van t Hoff factor of a 0.005 M aqueous solution of KCl is 1.95. Th sales, Solved What is the value for each of the van t Hoff factor Chegg sales, SOLVED What is the value of the van t Hoff factor for KCl if a sales, What is the value of Van t Hoff factor i for KCl if it is 80 dissociated in aqueous solution sales, faktor van t hoff KCL sales, SOLVED What is the ideal van t Hoff factor for the following sales, Solved Part A The van t Hoff factor for KCI is 1.85. What is sales, Odia What is the value of Van t Hoff factor i for KCl if it is 80 sales, SOLVED The values of Van t Hoff factors for KCl NaCl and K2SO4 sales, ee values of Van t Hoff factors for KCl NaCl and K2 SO4 sales, SOLVED The van t Hoff factor for KCl is i 1.85 . What is the sales, 57. If 0.2 M aq. solution of KCI is isotonic with 0.2 MK SO at sales, Problem 2.10 0.2 m aqueous solution of KCl freezes at 0.6800C sales, 6 Use log table necessary. Use of calculator is not allowed sales, van t Hoff factor i of a 0.5 w W aqueous solution of KCl sales, 1033.6 cm 3. A 0.5 aqueous solution of KCl was found to freeze sales, SOLVED Identify the solute with the lowest van t Hoff factor sales, Out of 0.1 molal aqueous solution of glucose and 0.1 molal aqueous sales, The values of van t Hoff factors for KCl NaCl and K 2SO 4 respectively are sales.
-
Next Day Delivery by DPD
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.