Van't hoff factor sales for potassium chloride
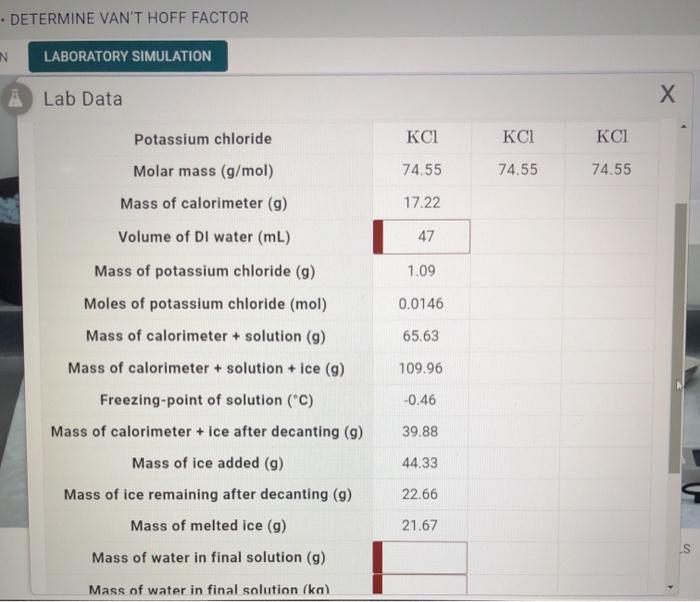
The values of van t Hoff factors KCl NaCl and K 2SO 4 sales, Solved DETERMINE VAN T HOFF FACTOR LABORATORY SIMULATION A Chegg sales, The van t Hoff factor of a 0.005 M aqueous solution of KCl is 1.95 sales, SOLVED DETERMINE VAN T HOFF FACTOR Lab Data Quantity Title TCh sales, Van t Hoff factor is maximum in KCl 50 ionised K SO 40 ionised sales, In which case van t Hoff factor is maximum a Potassium chloride sales, Solved RY. DETERMINE VAN T HOFF FACTOR Lab Data rieezing Chegg sales, SOLVED Lab Data X Solution Solution 2 Solution 3 Potassium sales, Solved DETERMINE VAN T HOFF FACTOR LABORATORY SIMULATION A Chegg sales, A 0.6 aqueous solution of KCl mol. wt. 74.5 freezes at 0.2 sales, ALEKS Calculating and using the van t Hoff factor for sales, SOLVED Freezing point depression constant C m Freezing point sales, ALEKS Calculating and using the van t Hoff factor for electrolytes 1 of 2 sales, SOLVED 25. The observed and calculated molar mass of KCl is 38.75 sales, Solved van t hoff factor the answer is not 2 Chegg sales, A 0.5 aqueous solution of KCl was found to freeze at 0.24 C. Calc sales, In which case van t Hoff factor i remains unchanged a PtCl4 sales, Solved the same van t Hoff factor.Potassium chloride a Chegg sales, SOLVED Determine the value of the van t Hoff factor i for the sales, Solved In which of the following pairs do both compounds Chegg sales, SOLVED Yois N Hlw Question This chloride vant Hoff factor sales, OneClass 63. Use the van t Hoff factors in Table 12.7 to sales, Answered What is the Van t Hoff Factor of the bartleby sales, Van t Hoff factor is maximum in KCl 50 ionised K SO 40 ionised sales, OneClass PART B Use the van t Hoff factors in the table below to sales, Electrolytes van t Hoff Factor sales, Q5 26 June Shift 1 A 0.5 percent solution of potassium chloride sales, SOLVED What is the van t Hoff factor for potassium chloride KCl sales, Out of 0.1 molal aqueous solution of glucose and 0.1 molal aqueous sales, Solved Question 14 6.66667 points For which of the Chegg sales, A 0.5 percent solution of potassium chloride was found to freeze at sales, 85 van t Hoff factor will be minimum for Filo sales, van t hoff factor for SrCl2 at 0.01 M is 1.6 Percent dissociation sales, At which temperature do solutions chloride and potassium chloride sales, CHEM 201 Calculating Actual van t Hoff Factor sales, Chemistry Vant Hoff s Factor Calculation by Elevation in Boiling sales, Out of 1M urea solution and 1M potassium chloride solution which sales, Chemistry Not Mystery Van t Hoff Factor reason to abnormal sales, Amount of salts calcium chloride CaCl 2 sodium chloride NaCl sales, Solved A saturated solution of potassium chloride in water Chegg sales.
- van't hoff factor for potassium chloride
- van't hoff factor for sodium bicarbonate
- van't hoff factor for sodium carbonate
- van't hoff factor for sodium nitrate
- van't hoff factor for sodium chloride
- van't hoff factor for sodium dichromate
- van't hoff factor for sodium sulfate
- van't hoff factor for srcl2
- van't hoff factor for strong electrolytes
- van't hoff factor for sugar in water
-
Next Day Delivery by DPD
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.