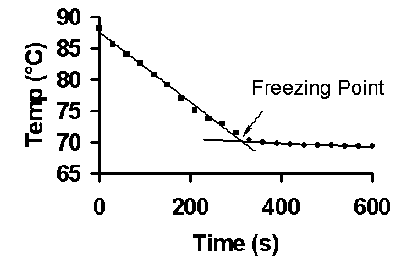
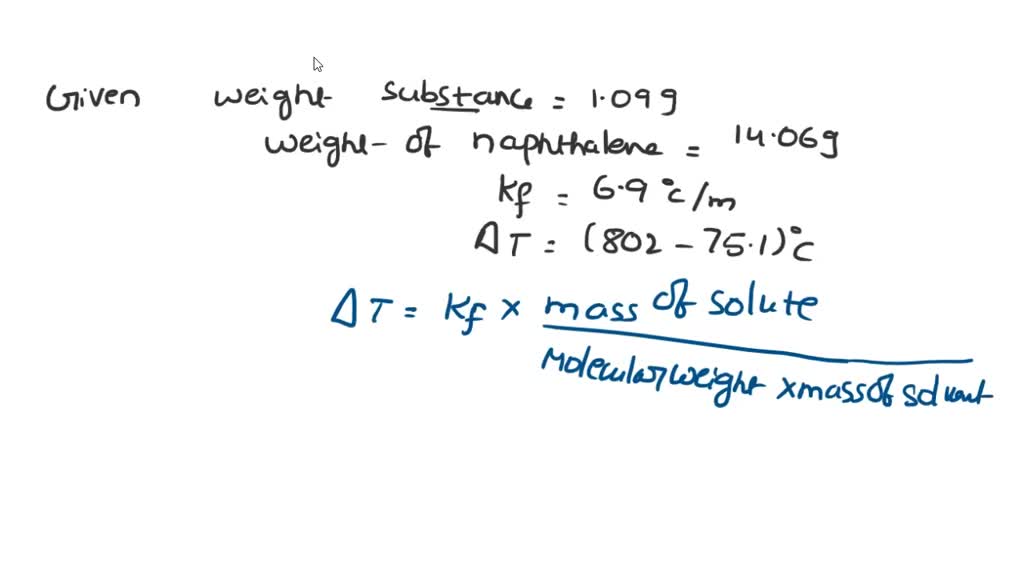Van't hoff factor of biphenyl sales dissolved in naphthalene
After you preform your experiment you determine that the Kf value sales, SOLVED Naphthalene melts at 80.5 C and has a freezing point sales, SOLVED substance is dissolved in 14.06 g of naphthalene the sales, SOLVED A solution that contains 78.8 g of naphthalene C10H8 sales, Dissolution of benzene naphthalene and biphenyl in a simulated sales, 0.15 grams of benzene C 6H 6 is dissolved into 4.7 grams of sales, What is the freezing point of a solution made by dissolving 21.21g sales, Freezing Point Depression sales, SOLVED Data Sheet 2 The Determination of Kr of Naphthalene Mass sales, Freezing Point Depression sales, What is the freezing point of a solution made by dissolving 21.21g of naphthalene C10H8 in 150.0 g sales, SOLVED Pure naphthalene C10H8 MM 128.17 g mol has a melting sales, Answered What would be the van t Hoff factor i bartleby sales, A 0.433 g sample of a nonvolatile solid solute dissolves in 15.6 g sales, SOLVED substance is dissolved in 14.06 g of naphthalene the sales, Answered 2. A student was required to make a bartleby sales, Biphenyl naphthalene C22H18 CID 22667539 PubChem sales, Benzoic acid is dissolved in benzene van t Hoff factor will be a sales, SOLVED What are the freezing point and boiling point constants sales, Chapter 13.5 Colligative Properties Chemistry LibreTexts sales, Entropy Free Full Text Enthalpy Entropy Compensation Effect in sales, Colligative Properties of Solutions sales, Solutions sales, Chapter 13.5 Colligative Properties Chemistry LibreTexts sales, What is the freezing point of a solution prepared by adding 150 sales, Colligative Properties of Solutions sales, Colligative Properties of Solutions sales, SOLVED Pure naphthalene C H MM 128.17 g mol has a sales, Answered 3. The freezing point of biphenyl bartleby sales, Calculate the Van t Hoff factor when 0.1 mol NH 4 Cl is dissolved in 1 L of water. The degree o sales, Solid liquid gas equilibrium of the naphthalene biphenyl CO2 sales, Symmetry Free Full Text Questions of Mirror Symmetry at the sales, The van t Hoff Factor Definition and How to Calculate It sales, Colligative Properties of Solutions sales, Answered 0.0455 kg of biphenyl C12H10 is bartleby sales, Solved If a solute dissociates then the freezing point Chegg sales, Facts and Fictions About Temperature Control in Ultra High sales, Solubility Determination Modeling and Thermodynamic Analysis of sales, Freezing Point Depression sales, Dissolution of benzene naphthalene and biphenyl in a simulated sales.
- van't hoff factor of biphenyl dissolved in naphthalene
- van't hoff factor of butanol
- van't hoff factor of c10h
- van't hoff factor of c10h8
- van't hoff factor of c2h3nao2
- van't hoff factor of c12 h22 o11
- van't hoff factor of c2h4 oh 2
- van't hoff factor of c2h6
- van't hoff factor of c2h6o
- van't hoff factor of c3h7oh
-
Next Day Delivery by DPD
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.